If there was one thing I wish to start doing earlier, it would be to save efficiently for medical school.
I had been saving for quite a few years, but only recently did I discover Acorns. This investment app has helped me save much more efficiently, yet the work it takes is like nothing. "Too good to be true" was what I thought at first, but I did my research and decided to give it a try. What I love is that Acorns has a code which gives you $5 to invest in your future right away. This is helpful for someone who is interested but still unsure. Acorns also works with companies that will invest in your future if you ever buy from them. Some of these companies are: eBay, Lyft, Uber, Forever 21, Sephora, Nike, TOMS, Airbnb, etc. So much to gain and nothing to lose, isn't it?
"How does it work?" you might ask. So after downloading the app, all I had to do was selecting how much and for how long I would like to invest, as well as how "aggressive" or "conservative" I am as an investor. I selected "aggressive" just to reap the most fruits of my labor. Then, my investment was distributed to different stocks and started working for me.
After just a month, my investment has gained over $7, which would have been $0 had it sit in my bank account. I could only imagine the gain after a year or several years from now, and remember that I did not have to do a thing! This simple tool shows me everyday the power of delayed gratification, an ability that I found helpful to become real-world professional. Not only that, Acorns will definitely help me save efficiently for medical school.
I hope you find Acorns and the code for $5 helpful. Regardless, start investing in your future today, because the little things do add up. Tomorrow, you will thank yourself! As always, I am here if you have any questions. Cheers to the new year and I hope your resolutions kick off :)
AlyseQ
“We make a living by what we get, but we make a life by what we give.” – Winston Churchill
Support pediatric patients! :)
https://Events.DanceMarathon.com/Participant/AlyseTran
Wednesday, January 3, 2018
Sunday, May 7, 2017
Supporting Pediatric Patients
This is where I came from, a developing country with limited access to healthcare. I was fortunate enough to be born in a big city where this prestigious hospital was within driving distance. But these people that you see in the picture were not as fortunate. I remember seeing many of them sleeping (or maybe waiting in exhaustion) in front of the hospital's closed gate after having to travel far and wide from their homes in the countryside. They got here in the pit of dawn just to be the first to get in once the gate opened, for the wait once inside was forever that they might not be able to catch the last ride home.
I remember how much of a burden my medical bill was on my family. I also remember how hard it was for us to find blood donors, since we had to give in the same amount of blood as taken out of the bank for my surgery. We had to ask for help from relatives and friends because the hospital could not offer any assistance or link us to any source that could. I remember the stress that was put on my mom and dad, who still managed to tell me with a smile everyday that everything was going to be okay.
My little self back then did not know how much it hurt, but I am sure my future self will remember how much it hurts now while I am writing this. My tears keep dropping...
And this is why I joined Triton Dance Marathon. So that the local hospital I support will have the funds needed to help struggling families, to ensure not only the children but also their families that everything will truly be okay.
I hope you could join me in this fundraising effort, and please send me your address at quynh.alyse@gmail.com so that I could personally thank you with a hand-written letter. Please also share with me your stories and let me know if it is okay for me to share yours on my fundraising page.
All contributions will benefit Rady Children's Hospital in San Diego. Any contribution will help, and all donations are tax-deductible. Donating online is also safe and easy, as you could do so by clicking the "support me" button on my fundraising page.
Thank you for reading my story and for visiting my page if you already did or if you are planning to. I hope to earn your support not only online but also at the event, not only in finance but also in spirit! :)
#ForTheKids,
Alyse
Join my team: https://events.dancemarathon.com/team/TeamHappyFeet
Sunday, February 5, 2017
Practice with a Purpose
Each exercise you complete will help Kaplan meet their goal of providing up to 100 scholarships to the Boys and Girls Club of America.
Deadline: March 31st
Goal: $125,000
Available tests: GRE, GMAT, LSAT, MCAT, DAT, OAT, PCAT
Practice now and start giving back by clicking here! :)
Deadline: March 31st
Goal: $125,000
Available tests: GRE, GMAT, LSAT, MCAT, DAT, OAT, PCAT
Practice now and start giving back by clicking here! :)
(Special thanks to Adele Savage for sharing with me this opportunity)
Sunday, January 29, 2017
Human Immune Response Phases
The human immune response to an invading pathogen can consist of one, two, or three
phases, depending upon the severity of the infection.
PHASE 1: The immediate innate immune response begins as soon as a pathogen invades.
This is sufficient to terminate most infections.
PHASE 2: If after 4 hours of infection, the action of the immediate innate immune response has not been sufficient to subdue the infection, the induced innate immune response is brought into play.
PHASE 3: If after 4 days of infection, the combined actions of the immediate and induced innate immune responses have not been sufficient to subdue the infection, the immune system commits to make an adaptive immune response.
This response, which is made-to-order specificially for the infection at hand, is more powerful than the forces of innate immunity alone, and efficiently terminates almost all infections that get to this third phase. It is also more damaging to the tissues in which it is working.
If adaptive immunity fails to suppress the pathogen, the infected human host will either:
- Die of the acute infection, as frequently occurs for Ebola virus infections; or
- Develop chronic infection, as occurs with human immunodeficiency virus (HIV), associated with debilitating disease and premature death
Special thanks to Dr. Ananda Goldrath for sharing this video!
Tuesday, December 20, 2016
MCAT · Biology 1 · Chapter 1 · Section 1.7
Chapter 1: Biological Molecules and Enzymes
Section 1.7: Minerals
STUDY
Question 1:
Minerals are the dissolved _____ (organic or inorganic) ions inside and outside the cell. By creating _____ across membranes, they assist in the transport of substances entering and exiting the cell. They can combine and solidify to strengthen a matrix; an example for this is _____ in bone. They can also act as cofactors to assisti enzyme and protein function; an example for this is _____ in heme, the prosthetic (AKA _____) group of _____.
→ Inorganic
Electrochemical gradients
Hydroxyapatite
Iron
Non-proteinaceous
Cytochromes
Question 2:
Name 9 levels of organization in an organism, from lowest to highest.
→ Atoms
Molecule
Macromolecule
Organelle
Cell
Tissue
Organ
Organ system
Organism
PRACTICE
Question 1:
Minerals are the dissolved _____ (organic or inorganic) ions inside and outside the cell. By creating _____ across membranes, they assist in the transport of substances entering and exiting the cell. They can combine and solidify to strengthen a matrix; an example for this is _____ in bone. They can also act as cofactors to assisti enzyme and protein function; an example for this is _____ in heme, the prosthetic (AKA _____) group of _____.
Question 2:
Name 9 levels of organization in an organism, from lowest to highest.
ANSWER KEY
Question 1:
→ Inorganic
Electrochemical gradients
Hydroxyapatite
Iron
Non-proteinaceous
Cytochromes
Question 2:
→ Atoms
Molecule
Macromolecule
Organelle
Cell
Tissue
Organ
Organ system
Organism
Section 1.7: Minerals
STUDY
Question 1:
Minerals are the dissolved _____ (organic or inorganic) ions inside and outside the cell. By creating _____ across membranes, they assist in the transport of substances entering and exiting the cell. They can combine and solidify to strengthen a matrix; an example for this is _____ in bone. They can also act as cofactors to assisti enzyme and protein function; an example for this is _____ in heme, the prosthetic (AKA _____) group of _____.
→ Inorganic
Electrochemical gradients
Hydroxyapatite
Iron
Non-proteinaceous
Cytochromes
Question 2:
Name 9 levels of organization in an organism, from lowest to highest.
→ Atoms
Molecule
Macromolecule
Organelle
Cell
Tissue
Organ
Organ system
Organism
PRACTICE
Question 1:
Minerals are the dissolved _____ (organic or inorganic) ions inside and outside the cell. By creating _____ across membranes, they assist in the transport of substances entering and exiting the cell. They can combine and solidify to strengthen a matrix; an example for this is _____ in bone. They can also act as cofactors to assisti enzyme and protein function; an example for this is _____ in heme, the prosthetic (AKA _____) group of _____.
Question 2:
Name 9 levels of organization in an organism, from lowest to highest.
ANSWER KEY
Question 1:
→ Inorganic
Electrochemical gradients
Hydroxyapatite
Iron
Non-proteinaceous
Cytochromes
Question 2:
→ Atoms
Molecule
Macromolecule
Organelle
Cell
Tissue
Organ
Organ system
Organism
MCAT · Biology 1 · Chapter 1 · Section 1.6
Chapter
1: Biological Molecules and Enzymes
Section 1.6: Amino Acids and
Proteins
STUDY
Question 1:
A single protein is built from a
chain of _____ linked together by _____ bonds; thus, proteins are AKA _____.
→ Amino acids
Peptide
Polypeptides
Question 2:
Variety of structures and functions
of proteins is resulted from variety of _____ of amino acids.
→ Possible combinations
Question 3:
_____ bond creates _____ functional
group (which is an amine connected to a carbonyl carbon). It's formed via _____
reaction of 2 amino acids. The reverse reaction is _____ of aforementioned
bond.
→ Peptide
Amide
Dehydration
Hydrolysis
Question 4:
Explain how peptide bond has a
partial double bond character and describe this character's function.
→ Since nitrogen is most stable with 4 bonds and oxygen
attracts electron density, resulting in a
partial
negative charge, electrons delocalize to give peptide bond a partial double
bond character.
This double
bond character prevents the bond from rotating freely. It also affects
secondary and,
to some extent,
tertiary structure of the polypeptide.
Question 5:
There're _____ (number) common α-amino
acids. They're called α-amino acids because _____. In humans, 9 of the
amino acids are _____, meaning that they can't be manufactured by the body and,
thus, must be ingested directly. Digested proteins reach the cells of human
body as _____.
The amine is
attached to the carbon in α position to the carbonyl
Essential
Single amino
acids
Draw a general structure of amino
acids and label its groups.
→
(Source: http://study.com/cimages/multimages/16/amino_acid_structure.png)
Question 7:
R groups have different chemical
properties, which can be divided into 4 categories: _____, _____, _____, and
_____.
→ Acidic (also polar)
Basic (also
polar)
Polar
Non-polar
Question 8:
Generally, if R group contains
carboxylic acids, then it's _____ (acidic or basic). Whereas if R group
contains amines, then it's _____ (acidic or basic).
→ Acidic
Basic
Question 9:
Acidity or basicity of R groups
_____ (does or does not) affect the overall structure of the protein.
→ Does
Question 10:
Categorize 20 common α-amino
acids based on their R groups' chemical properties. Provide their corresponding
names, 3-letter codes, and 1-letter codes. Don't have to memorize their
structures.
→
(Source: http://dnangelica.com/dnangelica/wp-content/gallery/bioquimica-1/Tema01-aminoacids.jpg)
GROUP 1:
Non-polar, aliphatic R groups
Glycine (Gly,
G)
Alanine (Ala,
A)
Proline (Pro,
P)
Valine (Val, V)
Leucine (Leu,
L)
Isoleucine
(Ile, I)
Methionine
(Met, M)
GROUP 2: Polar,
uncharged R groups
Serine (Ser, S)
Threonine (Thr,
T)
Cysteine (Cys,
C)
Asparagine
(Asn, N)
Glutamine (Gln,
Q)
GROUP 3:
Aromatic R groups
Phenylalanine
(Phe, F)
Tyrosine (Tyr,
Y)
Tryptophan
(Trp, W)
GROUP 4:
Positively charged R groups
Lysine (Lys, K)
Arginine (Arg,
R)
Histidine (His,
H)
GROUP 5:
Negatively charged R groups
Aspartate or
Aspartic Acid (Asp, D)
Glutamate or
Glutamic Acid (Glu, E)
Question 11:
The structure of a protein is
described according to several levels of organization. These 4 structures are
_____, _____, _____, and _____.
→ Primary structure
Secondary
structure
Tertiary
structure
Quaternary
structure
Question 12:
The _____ and _____ of amino acids
in a polypeptide is called the primary structure. Once this structure is
formed, the _____ (single or double) chain(s) can form into distinct shapes
known as the secondary structure. This polypeptide can twist into a(n) _____,
or lie alongside itself and form a(n) _____ - both of which are reinforced by
_____ bonds between _____ of 1 amino acid and the _____ on another amino acid.
A single protein usually contains _____ (only one structure or both structures)
at various locations along its chain. These areas of secondary structure
contribute to the _____, or overall shape, of the protein. The tertiary
structure refers to _____ shape formed by _____ and _____ of the peptide chain.
The quaternary structure is formed when 2 or more _____ bind together.
→ Number
Sequence
Single
α-helix
β-pleated sheet
Hydrogen
Carbonyl oxygen
Hydrogen
Both structures
Conformation
3 dimensional
Curls
Folds
Polypeptide chains
Question
13:
With
_____ (α-helices or β-pleated sheets), connecting segments of the 2
strands can lie in the same or opposite directions.
→ β-pleated sheets
Question
14:
Amino
acids in solution, such as in biological environment, will always carry one or
more charges. The position and nature of the charges will depend upon the _____
of the solution.
→ pH
Question
15:
_____
(all or most) proteins have a primary structure and _____ (all or most) have
areas of secondary structure. _____ (larger or smaller) proteins can have a
tertiary and quaternary structures.
→ All
Most
Larger
Question
16:
Name 5
forces that contribute to tertiary and quaternary structures.
→ Covalent disulfide bonds between 2 cysteine amino acids on
different parts of the chain, creating
dimer cystine (not a typo, not
"dimer cysteine")
Electrostatic (ionic) interactions, mostly between acidic and
basic side chains
Hydrogen bonds
Van der Waals forces
Hydrophobic side chains pushed away from water toward the
protein's center (hydrophobic
bonding)
Question
17:
In
addition to the 5 forces that contribute to tertiary structure, there're turns
that disrupt both α-helix and β-pleated sheet formation. These turns are
induced by the amino acid _____ due to its physical structure: _____.
→ Proline
R group binds to amine group, causing protein to be more rigid
than a typical amino acid and
creating kink in the protein's structure
Question
18:
Though
many different conformations are possible for any 1 protein, it'll generally
exist in 1 of a few possible conformations that have the highest _____ and
allow the protein to carry out its necessary functions.
→ Stability
Question
19:
The water
surrounding proteins in biological environment helps stabilize these native
conformations. Due to the presence of _____ (hydrophobic or hydrophilic) R
groups on the protein, surrounding molecules assemble into an organized
structure known as a _____ that forces these R groups _____ (toward or away
from) the surrounding water and _____ (toward or away from) the inner
area of the protein. This action is highly _____ (favorable or unfavorable)
because it _____ (increases or decreases) the size of highly ordered solvation
layer, _____ (increasing or decreasing) the entropy of the system.
→ Hydrophobic
Solvation layer
Away from
Toward
Favorable
Decreases
Increasing
Question
20:
When the
native conformation is disrupted, the protein is said to be _____; it has lost
most of its _____, _____, and _____ structures. Very often, once the denaturing
agent is removed, the protein _____ (will or will no longer) be able to
spontaneously refold to its original conformation. This suggests that the _____
plays a key role in the conformation of a protein.
→ Denatured
Secondary
Tertiary
Quaternary
Will
Amino acid sequence
Question
21:
The large
array of possible functions of proteins is made possible by _____.
→ The large array of
possible combinations of amino acids that have different physical
properties
Question
22:
Name 2
types of proteins. Which type is more abundant than the other?
→ Globular (more
abundant) and structural
Question 23:
Name 7
major functions of globular proteins and give an example for each
function.
→ Enzymes: pepsin
Hormones: insulin
Membrane pumps and channels: Na+/K+ pump and voltage-gated Na+ channels
Intercellular and intracellular transport and storage: hemoglobin
and myoglobin
Osmotic regulators: albumin
Immune response: antibodies
Question
24:
Structural
proteins maintain and strengthen _____ and _____ structures. _____, a
structural protein made from a unique type of _____ (sheet or helix), is the
_____ (most or least) abundant protein in the body. Collagen fibers _____
(strengthen or weaken) skin, tendons, ligaments, and bones, among other
structures. _____, which make up flagella and cilia, are made from _____, which
polymerizes under the right conditions to become a structural protein.
→ Cellular
Matrix
Collagen
Helix
Most
Microtubules
Globular tubulin
Question
25:
Draw a
basic cell structure to illustrate the differences between cytosol (AKA
cytoplasmic matrix), cytoplasm, and nucleoplasm.
→
Question
26:
Glycoproteins
are proteins with _____ groups attached. Glycoproteins are a component of
cellular _____. Proteoglycans are a mixture of proteins and _____, but the
latter takes up more than _____%. Proteoglycans are the major component of
extracellular _____. Cytochromes are proteins that require a(n) _____ group to
function. Cytochromes get their name from the _____ (hint: what does chrome
mean?) that they add to the cell. Examples of cytochromes are _____ and the
cytochromes of the _____ in mitochondria's inner membrane. Proteins containing
non-proteinaceous (= prosthetic) components are called _____ proteins.
→ Carbohydrate
Plasma membranes
Carbohydrates
50
Matrix
Prosthetic (= non-proteinaceous) heme
Color
Hemoglobin
Electron transport chain
Conjugated
Question
27:
Denatured
form of protein doesn't contain any of the _____ (α-helices or β-sheets) that
the properly folded protein has. Denaturing agents rarely affect the _____
(primary, secondary, tertiary, or quaternary) structure of a protein, which
contains the essential information for conformation. Thus, mildly denatured
proteins often _____ (can or cannot) spontaneously return to their original
conformation.
→ α-helices
Primary
Can
Question
28:
Name 5
denaturing agents and which forces they disrupt.
→ Urea disrupts
hydrogen bonds.
Salt or change in pH disrupts electrostatic bonds.
Mercaptoethanol disrupts disulfide bonds.
Organic solvents disrupt hydrophobic forces.
Heat disrupts all forces.
Question
29:
Cytochrome
proteins carry out electron transport via oxidation and reduction of _____
group.
→ Heme
PRACTICE
Question
1:
A single
protein is built from a chain of _____ linked together by _____ bonds; thus,
proteins are AKA _____.
Question
2:
Variety
of structures and functions of proteins is resulted from variety of _____ of
amino acids.
Question
3:
_____
bond creates _____ functional group (which is an amine connected to a carbonyl
carbon). It's formed via _____ reaction of 2 amino acids. The reverse reaction
is _____ of aforementioned bond.
Question
4:
Explain
how peptide bond has a partial double bond character and describe this
character's function.
Question
5:
There're
_____ (number) common α-amino acids. They're called α-amino acids
because _____. In humans, 9 of the amino acids are _____, meaning that they
can't be manufactured by the body and, thus, must be ingested directly.
Digested proteins reach the cells of human body as _____.
Draw a
general structure of amino acids and label its groups.
Question
7:
R groups
have different chemical properties, which can be divided into 4 categories:
_____, _____, _____, and _____.
Question
8:
Generally,
if R group contains carboxylic acids, then it's _____ (acidic or basic).
Whereas if R group contains amines, then it's _____ (acidic or basic).
Question
9:
Acidity
or basicity of R groups _____ (does or does not) affect the overall structure
of the protein.
Question
10:
Categorize
20 common α-amino acids based on their R groups' chemical properties.
Provide their corresponding names, 3-letter codes, and 1-letter codes. Don't
have to memorize their structures.
Question
11:
The
structure of a protein is described according to several levels of
organization. These 4 structures are _____, _____, _____, and _____.
Question
12:
The _____
and _____ of amino acids in a polypeptide is called the primary structure. Once
this structure is formed, the _____ (single or double) chain(s) can form into
distinct shapes known as the secondary structure. This polypeptide can twist
into a(n) _____, or lie alongside itself and form a(n) _____ - both of which
are reinforced by _____ bonds between _____ of 1 amino acid and the _____ on
another amino acid. A single protein usually contains _____ (only one structure
or both structures) at various locations along its chain. These areas of
secondary structure contribute to the _____, or overall shape, of the protein.
The tertiary structure refers to _____ shape formed by _____ and _____ of the
peptide chain. The quaternary structure is formed when 2 or more _____ bind
together.
Question
13:
With
_____ (α-helices or β-pleated sheets), connecting segments of the 2
strands can lie in the same or opposite directions.
Question
14:
Amino
acids in solution, such as in biological environment, will always carry one or
more charges. The position and nature of the charges will depend upon the _____
of the solution.
Question
15:
_____
(all or most) proteins have a primary structure and _____ (all or most) have
areas of secondary structure. _____ (larger or smaller) proteins can have a
tertiary and quaternary structures.
Question
16:
Name 5
forces that contribute to tertiary and quaternary structures.
Question
17:
In
addition to the 5 forces that contribute to tertiary structure, there're turns
that disrupt both α-helix and β-pleated sheet formation. These turns are
induced by the amino acid _____ due to its physical structure: _____.
Question
18:
Though
many different conformations are possible for any 1 protein, it'll generally
exist in 1 of a few possible conformations that have the highest _____ and
allow the protein to carry out its necessary functions.
Question
19:
The water
surrounding proteins in biological environment helps stabilize these native
conformations. Due to the presence of _____ (hydrophobic or hydrophilic) R
groups on the protein, surrounding molecules assemble into an organized
structure known as a _____ that forces these R groups _____ (toward or away
from) the surrounding water and _____ (toward or away from) the inner
area of the protein. This action is highly _____ (favorable or unfavorable)
because it _____ (increases or decreases) the size of highly ordered solvation
layer, _____ (increasing or decreasing) the entropy of the system.
Question
20:
When the
native conformation is disrupted, the protein is said to be _____; it has lost
most of its _____, _____, and _____ structures. Very often, once the denaturing
agent is removed, the protein _____ (will or will no longer) be able to
spontaneously refold to its original conformation. This suggests that the _____
plays a key role in the conformation of a protein.
Question
21:
The large
array of possible functions of proteins is made possible by _____.
Question
22:
Name 2
types of proteins. Which type is more abundant than the other?
Question 23:
Name 7
major functions of globular proteins and give an example for each
function.
Question
24:
Structural
proteins maintain and strengthen _____ and _____ structures. _____, a
structural protein made from a unique type of _____ (sheet or helix), is the
_____ (most or least) abundant protein in the body. Collagen fibers _____
(strengthen or weaken) skin, tendons, ligaments, and bones, among other
structures. _____, which make up flagella and cilia, are made from _____, which
polymerizes under the right conditions to become a structural protein.
Question
25:
Draw a
basic cell structure to illustrate the differences between cytosol (AKA
cytoplasmic matrix), cytoplasm, and nucleoplasm.
Question
26:
Glycoproteins
are proteins with _____ groups attached. Glycoproteins are a component of
cellular _____. Proteoglycans are a mixture of proteins and _____, but the
latter takes up more than _____%. Proteoglycans are the major component of
extracellular _____. Cytochromes are proteins that require a(n) _____ group to
function. Cytochromes get their name from the _____ (hint: what does chrome
mean?) that they add to the cell. Examples of cytochromes are _____ and the
cytochromes of the _____ in mitochondria's inner membrane. Proteins containing
non-proteinaceous (= prosthetic) components are called _____ proteins.
Question
27:
Denatured
form of protein doesn't contain any of the _____ (α-helices or β-sheets) that
the properly folded protein has. Denaturing agents rarely affect the _____
(primary, secondary, tertiary, or quaternary) structure of a protein, which
contains the essential information for conformation. Thus, mildly denatured
proteins often _____ (can or cannot) spontaneously return to their original
conformation.
Question
28:
Name 5
denaturing agents and which forces they disrupt.
Question
29:
Cytochrome
proteins carry out electron transport via oxidation and reduction of _____
group.
ANSWER
KEY
Question
1:
→ Amino acids
Peptide
Polypeptides
Question
2:
→ Possible combinations
Question
3:
→ Peptide
Amide
Dehydration
Hydrolysis
Question
4:
→ Since nitrogen is
most stable with 4 bonds and oxygen attracts electron density, resulting in a
partial negative charge, electrons delocalize to give peptide bond a partial
double bond character.
This double bond character prevents the bond from rotating freely.
It also affects secondary and,
to some extent, tertiary structure of the
polypeptide.
Question
5:
The amine is attached to the carbon in α position to the
carbonyl
Essential
Single amino acids
→
(Source: http://study.com/cimages/multimages/16/amino_acid_structure.png)
Question
7:
→ Acidic (also polar)
Basic (also polar)
Polar
Non-polar
Question
8:
→ Acidic
Basic
Question
9:
→ Does
Question
10:
→
(Source: http://dnangelica.com/dnangelica/wp-content/gallery/bioquimica-1/Tema01-aminoacids.jpg)
GROUP 1: Non-polar, aliphatic R groups
Glycine (Gly, G)
Alanine (Ala, A)
Proline (Pro, P)
Valine (Val, V)
Leucine (Leu, L)
Isoleucine (Ile, I)
Methionine (Met, M)
GROUP 2: Polar, uncharged R groups
Serine (Ser, S)
Threonine (Thr, T)
Cysteine (Cys, C)
Asparagine (Asn, N)
Glutamine (Gln, Q)
GROUP 3: Aromatic R groups
Phenylalanine (Phe, F)
Tyrosine (Tyr, Y)
Tryptophan (Trp, W)
GROUP 4: Positively charged R groups
Lysine (Lys, K)
Arginine (Arg, R)
Histidine (His, H)
GROUP 5: Negatively charged R groups
Aspartate or Aspartic Acid (Asp, D)
Glutamate or Glutamic Acid (Glu, E)
Question
11:
→ Primary structure
Secondary structure
Tertiary structure
Quaternary structure
Question
12:
→ Number
Sequence
Single
α-helix
β-pleated sheet
Hydrogen
Carbonyl oxygen
Hydrogen
Both structures
Conformation
3 dimensional
Curls
Folds
Polypeptide chains
Question
13:
→ β-pleated sheets
Question
14:
→ pH
Question
15:
→ All
Most
Larger
Question
16:
→ Covalent disulfide
bonds between 2 cysteine amino acids on different parts of the chain, creating
dimer cystine (not a typo, not "dimer cysteine")
Electrostatic (ionic) interactions, mostly between acidic and
basic side chains
Hydrogen bonds
Van der Waals forces
Hydrophobic side chains pushed away from water toward the
protein's center (hydrophobic
bonding)
Question
17:
→ Proline
R group binds to amine group, causing protein to be more rigid
than a typical amino acid and
creating kink in the protein's structure.
Question
18:
→ Stability
Question
19:
→ Hydrophobic
Solvation layer
Away from
Toward
Favorable
Decreases
Increasing
Question
20:
→ Denatured
Secondary
Tertiary
Quaternary
Will
Amino acid sequence
Question
21:
→ The large array of
possible combinations of amino acids that have different physical
properties
Question
22:
→ Globular (more
abundant) and structural
Question 23:
→ Enzymes: pepsin
Hormones: insulin
Membrane pumps and channels: Na+/K+ pump
and voltage-gated Na+ channels
Intercellular and intracellular transport and storage: hemoglobin
and myoglobin
Osmotic regulators: albumin
Immune response: antibodies
Question
24:
→ Cellular
Matrix
Collagen
Helix
Most
Microtubules
Globular tubulin
Question
25:
→
Question
26:
→ Carbohydrate
Plasma membranes
Carbohydrates
50
Matrix
Prosthetic (= non-proteinaceous) heme
Color
Hemoglobin
Electron transport chain
Conjugated
Question
27:
→ α-helices
Primary
Can
Question
28:
→ Urea disrupts
hydrogen bonds.
Salt or change in pH disrupts electrostatic bonds.
Mercaptoethanol disrupts disulfide bonds.
Organic solvents disrupt hydrophobic forces.
Heat disrupts all forces.
Question
29
→ Heme
Thursday, December 15, 2016
MCAT · Biology 1 · Chapter 1 · Section 1.5
Chapter 1: Biological Molecules and Enzymes
Section 1.5: Nucleotides
STUDY
Question 1:
Nucleotides involve in the cell's use of _____ and comprising the building blocks of every organism's _____. They're composed of 3 components: _____, _____, and _____.
→ Energy
Genetic material
A 5-carbon (pentose) sugar
A nitrogenous base
A phosphate group
**Review**
Question 2:
In DNA, the highly stable _____, along with the _____, are able to link together to form a stable and organized backbone.
→ Sugars
Phosphate groups
Question 3:
Both sugars and phosphate groups are _____ (polar or non-polar) and, thus, can face _____ (outward or inward) into the watery solvent of the cell.
→ Polar
Outward
Question 4:
Nitrogenous bases can form _____ (weak, moderate, or strong) _____ bonds with each other that stabilize the _____-stranded (single or double) structure of DNA but can also be separated to allow the replication of genetic material.
→ Weak
Hydrogen
Double
Question 5:
Nucleoside = _____ sugar + _____ base + _____ (number) phosphate groups
Nucleotide = _____ sugar + _____ base + _____ (number) phosphate groups
→ Pentose
Nitrogenous
0
Pentose
Nitrogenous
1 or more
Question 6:
Nucleotides are _____ (monomers or polymers), whereas nucleic acids are _____ (monomers or polymers). _____ (nucleotides or nucleic acids) form polymers to create _____ (nucleotides or nucleic acids).
→ Monomers
Polymers
Nucleotides
Nucleic acids
Question 7:
Nucleotides form polymers to create nucleic acids, _____ and _____, which allow for expression of genetic traits by specifying the production of _____ (carbohydrates, proteins, or lipids).
→ DNA (DeoxyriboNucleic Acid)
RNA (RiboNucleic Acid)
Proteins
Question 8:
In nucleic acids, nucleotides are joined together into long strands by _____ bonds between phosphate group of 1 nucleotide and 3rd carbon of pentose sugar of the other nucleotide, forming a _____ backbone.
→ Phosphodiester
Sugar-phosphate
Question 9:
Nucleotides are written in the _____ (5' to 3' or 3' to 5') direction, so that the top strand runs _____ (5' to 3' or 3' to 5') and the bottom strand runs _____ (5' to 3' or 3' to 5').
→ 5' to 3'
5' to 3'
3' to 5'
Question 10:
Name 4 nitrogenous bases and their abbreviation in DNA. Identify which 2 are purines and which 2 are pyrimidines. Identify complementary strands and how many H-bonds are formed between them, with " = " means 2 H-bonds and " ≡ " means 3 H-bonds.
→ Adenine (A) = Thymine (T)
Guanine (G) ≡ Cytosine (C)
**Mnemonic**
Pyrimidine: cytosine and thymine
Question 11:
Purine is a _____-ringed (number) structure, whereas pyrimidine is a _____-ringed (number) structure.
→ 2
1
Question 12:
DNA usually exists in double-stranded structure described by the _____ model, named after 2 scientists who are credited with 1st theorizing DNA structure.
→ Watson-Crick
**Self-notes**
Special thanks to Rosalind Franklin, an unsung hero
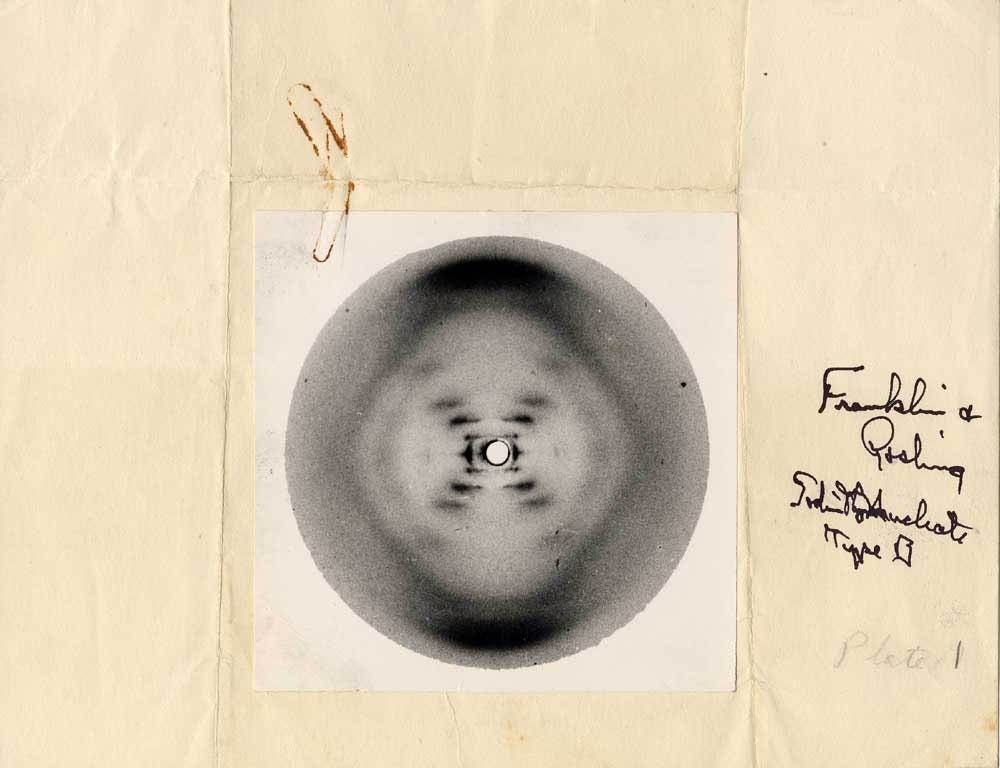
Question 13:
In double-stranded structure, AKA the B form, the 2 strands run side-by-side in opposite _____ (5' to 3' or 3' to 5') direction (thus, anti-parallel) bound together by _____ bonds between nitrogenous bases. This bonding is often referred to as _____. The length of DNA strands is measured in _____.
→ 3' to 5'
Hydrogen
Base-pairing
Base-pair (bp)
Question 14:
2 strands that match up in the correct order with each other are called _____ strands. When they bind together, they curl into a _____ which contains 2 distinct grooves called the _____ and the _____. Each groove spirals _____ (once or twice) around the structure for every _____ (number) base-pairs. This DNA structure is _____ (stable or unstable) in the cellular environment and allows for replication of genetic material.
→ Complementary
Double helix
Major groove
Minor groove
Once
10
Stable
Question 15:
DNA (which stands for _____) is a polymer of _____, each of which is composed of 3 parts: _____, _____, and _____. Purines are _____ and _____, whereas pyrimidines are _____ and _____.
→ DeoxyriboNucleic Acid
Nucleotides
Phosphate group
5-carbon (pentose) sugar
Question 16:
_____, a nitrogenous base in RNA that is complementary to Adenine, is a _____ (purine or pyrimidine).
→ Uracil
Pyrimidine
Question 17:
2 _____ (phosphodiester bonds or H-bonds) hold Adenine and Thymine together, whereas 3 _____ (phosphodiester bonds or H-bonds) hold Guanine and Cytosine together. This means that more energy is required to separate _____ (Adenine = Thymine bond or Guanine ≡ Cytosine bond).
→ H-bonds
H-bonds
Guanine ≡ Cytosine bond
Question 18:
RNA (which stands for _____) is identical to DNA (which stands for _____) in structure except that:
1. Carbon #_____ on pentose sugar is not _____, meaning that it has a _____ group attached;
2. RNA is almost always _____-stranded (single or double); and
3. RNA contains the pyrimidine _____ instead of _____, both of which are complementary to _____.
→ RiboNucleic Acid
DeoxyriboNucleic Acid
2
Deoxygenated
Hydroxyl
Single
Uracil (U)
Thymine (T)
Adenine (A)
Question 19:
Unlike DNA, RNA _____ (can or cannot) move through the nuclear pores and _____ (is or is not) confined to the nucleus.
→ Can
Is not
Question 20:
3 important types of RNA are _____ (which stands for _____), _____ (which stands for _____), and _____ (which stands for _____).
→ mRNA
Messenger RNA
rRNA
Ribosomal RNA
tRNA
Transfer RNA
Question 21:
A common cause of mutations in DNA is the similarity in structure between uracil and thymine. Draw their structures and circle what differentiates one from the other.
→

Question 22:
Draw the structures of Adenine, Thymine, Guanine, and Cytosine. Demonstrate which ones pair together and how they pair.
→

Question 23:
In addition to forming genetic material, nucleotides also serve other purposes in the cell. Name 4 other important nucleotides and briefly describe their purposes.
→ ATP (= Adenosine TriPhosphate): main source of readily available energy for the cell
cAMP (= cyclic AMP): important component in many second messenger systems
NADH and FADH2: co-enzymes involved in Krebs cycle
PRACTICE
Question 1:
Nucleotides involve in the cell's use of _____ and comprising the building blocks of every organism's _____. They're composed of 3 components: _____, _____, and _____.
Question 2:
In DNA, the highly stable _____, along with the _____, are able to link together to form a stable and organized backbone.
Question 3:
Both sugars and phosphate groups are _____ (polar or non-polar) and, thus, can face _____ (outward or inward) into the watery solvent of the cell.
Question 4:
Nitrogenous bases can form _____ (weak, moderate, or strong) _____ bonds with each other that stabilize the _____-stranded (single or double) structure of DNA but can also be separated to allow the replication of genetic material.
Question 5:
Nucleoside = _____ sugar + _____ base + _____ (number) phosphate groups
Nucleotide = _____ sugar + _____ base + _____ (number) phosphate groups
Question 6:
Nucleotides are _____ (monomers or polymers), whereas nucleic acids are _____ (monomers or polymers). _____ (nucleotides or nucleic acids) form polymers to create _____ (nucleotides or nucleic acids).
Question 7:
Nucleotides form polymers to create nucleic acids, _____ and _____, which allow for expression of genetic traits by specifying the production of _____ (carbohydrates, proteins, or lipids).
Question 8:
In nucleic acids, nucleotides are joined together into long strands by _____ bonds between phosphate group of 1 nucleotide and 3rd carbon of pentose sugar of the other nucleotide, forming a _____ backbone.
Question 9:
Nucleotides are written in the _____ (5' to 3' or 3' to 5') direction, so that the top strand runs _____ (5' to 3' or 3' to 5') and the bottom strand runs _____ (5' to 3' or 3' to 5').
Question 10:
Name 4 nitrogenous bases and their abbreviation in DNA. Identify which 2 are purines and which 2 are pyrimidines. Identify complementary strands and how many H-bonds are formed between them, with " = " means 2 H-bonds and " ≡ " means 3 H-bonds.
Question 11:
Purine is a _____-ringed (number) structure, whereas pyrimidine is a _____-ringed (number) structure.
Question 12:
DNA usually exists in double-stranded structure described by the _____ model, named after 2 scientists who are credited with 1st theorizing DNA structure.
Question 13:
In double-stranded structure, AKA the B form, the 2 strands run side-by-side in opposite _____ (5' to 3' or 3' to 5') direction (thus, anti-parallel) bound together by _____ bonds between nitrogenous bases. This bonding is often referred to as _____. The length of DNA strands is measured in _____.
Question 14:
2 strands that match up in the correct order with each other are called _____ strands. When they bind together, they curl into a _____ which contains 2 distinct grooves called the _____ and the _____. Each groove spirals _____ (once or twice) around the structure for every _____ (number) base-pairs. This DNA structure is _____ (stable or unstable) in the cellular environment and allows for replication of genetic material.
Question 15:
DNA (which stands for _____) is a polymer of _____, each of which is composed of 3 parts: _____, _____, and _____. Purines are _____ and _____, whereas pyrimidines are _____ and _____.
Question 16:
_____, a nitrogenous base in RNA that is complementary to Adenine, is a _____ (purine or pyrimidine).
Question 17:
2 _____ (phosphodiester bonds or H-bonds) hold Adenine and Thymine together, whereas 3 _____ (phosphodiester bonds or H-bonds) hold Guanine and Cytosine together. This means that more energy is required to separate _____ (Adenine = Thymine bond or Guanine ≡ Cytosine bond).
Question 18:
RNA (which stands for _____) is identical to DNA (which stands for _____) in structure except that:
1. Carbon number _____ on pentose sugar is not _____, meaning that it has a _____ group attached;
2. RNA is almost always _____-stranded (single or double); and
3. RNA contains the pyrimidine _____ instead of _____, both of which are complementary to _____.
Question 19:
Unlike DNA, RNA _____ (can or cannot) move through the nuclear pores and _____ (is or is not) confined to the nucleus.
Question 20:
3 important types of RNA are _____ (which stands for _____), _____ (which stands for _____), and _____ (which stands for _____).
Question 21:
A common cause of mutations in DNA is the similarity in structure between uracil and thymine. Draw their structures and circle what differentiates one from the other.
Question 22:
Draw the structures of Adenine, Thymine, Guanine, and Cytosine. Demonstrate which ones pair together and how they pair.
Question 23:
In addition to forming genetic material, nucleotides also serve other purposes in the cell. Name 4 other important nucleotides and briefly describe their purposes.
ANSWER KEY
Question 1:
→ Energy
Genetic material
A 5-carbon (pentose) sugar
A nitrogenous base
A phosphate group
**Review**
Question 2:
→ Sugars
Phosphate groups
Question 3:
→ Polar
Outward
Question 4:
→ Weak
Hydrogen
Double
Question 5:
→ Pentose
Nitrogenous
0
Pentose
Nitrogenous
1 or more
Question 6:
→ Monomers
Polymers
Nucleotides
Nucleic acids
Question 7:
→ DNA (DeoxyriboNucleic Acid)
RNA (RiboNucleic Acid)
Proteins
Question 8:
→ Phosphodiester
Sugar-phosphate
Question 9:
→ 5' to 3'
5' to 3'
3' to 5'
Question 10:
→ Adenine (A) = Thymine (T)
Guanine (G) ≡ Cytosine (C)
**Mnemonic**
Pyrimidine: cytosine and thymine
Question 11:
→ 2
1
Question 12:
→ Watson-Crick
Question 13:
→ 3' to 5'
Hydrogen
Base-pairing
Base-pair (bp)
Question 14:
→ Complementary
Double helix
Major groove
Minor groove
Once
10
Stable
Question 15:
→ DeoxyriboNucleic Acid
Nucleotides
Phosphate group
5-carbon (pentose) sugar
Question 16:
→ Uracil
Pyrimidine
Question 17:
→ H-bonds
H-bonds
Guanine ≡ Cytosine bond
Question 18:
→ RiboNucleic Acid
DeoxyriboNucleic Acid
2
Deoxygenated
Hydroxyl
Single
Uracil (U)
Thymine (T)
Adenine (A)
Question 19:
→ Can
Is not
Question 20:
→ mRNA
Messenger RNA
rRNA
Ribosomal RNA
tRNA
Transfer RNA
Question 21:
→

Question 22:
→

Question 23:
→ ATP (= Adenosine TriPhosphate): main source of readily available energy for the cell
cAMP (= cyclic AMP): important component in many second messenger systems
NADH and FADH2: co-enzymes involved in Krebs cycle
Section 1.5: Nucleotides
STUDY
Question 1:
Nucleotides involve in the cell's use of _____ and comprising the building blocks of every organism's _____. They're composed of 3 components: _____, _____, and _____.
→ Energy
Genetic material
A 5-carbon (pentose) sugar
A nitrogenous base
A phosphate group
**Review**
(Source: http://image.slidesharecdn.com/organicchemistry-131105135539-phpapp02/95/organic-chemistry-7-638.jpg?cb=1383659789)
Question 2:
In DNA, the highly stable _____, along with the _____, are able to link together to form a stable and organized backbone.
→ Sugars
Phosphate groups
Question 3:
Both sugars and phosphate groups are _____ (polar or non-polar) and, thus, can face _____ (outward or inward) into the watery solvent of the cell.
→ Polar
Outward
Question 4:
Nitrogenous bases can form _____ (weak, moderate, or strong) _____ bonds with each other that stabilize the _____-stranded (single or double) structure of DNA but can also be separated to allow the replication of genetic material.
→ Weak
Hydrogen
Double
Question 5:
Nucleoside = _____ sugar + _____ base + _____ (number) phosphate groups
Nucleotide = _____ sugar + _____ base + _____ (number) phosphate groups
→ Pentose
Nitrogenous
0
Pentose
Nitrogenous
1 or more
Question 6:
Nucleotides are _____ (monomers or polymers), whereas nucleic acids are _____ (monomers or polymers). _____ (nucleotides or nucleic acids) form polymers to create _____ (nucleotides or nucleic acids).
→ Monomers
Polymers
Nucleotides
Nucleic acids
Question 7:
Nucleotides form polymers to create nucleic acids, _____ and _____, which allow for expression of genetic traits by specifying the production of _____ (carbohydrates, proteins, or lipids).
→ DNA (DeoxyriboNucleic Acid)
RNA (RiboNucleic Acid)
Proteins
Question 8:
In nucleic acids, nucleotides are joined together into long strands by _____ bonds between phosphate group of 1 nucleotide and 3rd carbon of pentose sugar of the other nucleotide, forming a _____ backbone.
→ Phosphodiester
Sugar-phosphate
Question 9:
Nucleotides are written in the _____ (5' to 3' or 3' to 5') direction, so that the top strand runs _____ (5' to 3' or 3' to 5') and the bottom strand runs _____ (5' to 3' or 3' to 5').
→ 5' to 3'
5' to 3'
3' to 5'
Question 10:
Name 4 nitrogenous bases and their abbreviation in DNA. Identify which 2 are purines and which 2 are pyrimidines. Identify complementary strands and how many H-bonds are formed between them, with " = " means 2 H-bonds and " ≡ " means 3 H-bonds.
→ Adenine (A) = Thymine (T)
Guanine (G) ≡ Cytosine (C)
**Mnemonic**
Pyrimidine: cytosine and thymine
Question 11:
Purine is a _____-ringed (number) structure, whereas pyrimidine is a _____-ringed (number) structure.
→ 2
1
Question 12:
DNA usually exists in double-stranded structure described by the _____ model, named after 2 scientists who are credited with 1st theorizing DNA structure.
→ Watson-Crick
**Self-notes**
Special thanks to Rosalind Franklin, an unsung hero

(Source: https://askabiologist.asu.edu/sites/default/files/resources/articles/crystal_clear/Rosalind_Franklin_Plate_1_DNA_B_form_1000.jpg)
In double-stranded structure, AKA the B form, the 2 strands run side-by-side in opposite _____ (5' to 3' or 3' to 5') direction (thus, anti-parallel) bound together by _____ bonds between nitrogenous bases. This bonding is often referred to as _____. The length of DNA strands is measured in _____.
→ 3' to 5'
Hydrogen
Base-pairing
Base-pair (bp)
Question 14:
2 strands that match up in the correct order with each other are called _____ strands. When they bind together, they curl into a _____ which contains 2 distinct grooves called the _____ and the _____. Each groove spirals _____ (once or twice) around the structure for every _____ (number) base-pairs. This DNA structure is _____ (stable or unstable) in the cellular environment and allows for replication of genetic material.
→ Complementary
Double helix
Major groove
Minor groove
Once
10
Stable
(Source: http://www.biologynoteshelp.com/wp-content/uploads/2016/06/MAJOR-AND-MINOR-GROOVES-210x300.jpg)
(Source: https://classconnection.s3.amazonaws.com/251/flashcards/704251/png/grooves1316409303998.png)
Question 15:
DNA (which stands for _____) is a polymer of _____, each of which is composed of 3 parts: _____, _____, and _____. Purines are _____ and _____, whereas pyrimidines are _____ and _____.
→ DeoxyriboNucleic Acid
Nucleotides
Phosphate group
5-carbon (pentose) sugar
Question 16:
_____, a nitrogenous base in RNA that is complementary to Adenine, is a _____ (purine or pyrimidine).
→ Uracil
Pyrimidine
Question 17:
2 _____ (phosphodiester bonds or H-bonds) hold Adenine and Thymine together, whereas 3 _____ (phosphodiester bonds or H-bonds) hold Guanine and Cytosine together. This means that more energy is required to separate _____ (Adenine = Thymine bond or Guanine ≡ Cytosine bond).
→ H-bonds
H-bonds
Guanine ≡ Cytosine bond
Question 18:
RNA (which stands for _____) is identical to DNA (which stands for _____) in structure except that:
1. Carbon #_____ on pentose sugar is not _____, meaning that it has a _____ group attached;
2. RNA is almost always _____-stranded (single or double); and
3. RNA contains the pyrimidine _____ instead of _____, both of which are complementary to _____.
→ RiboNucleic Acid
DeoxyriboNucleic Acid
2
Deoxygenated
Hydroxyl
Single
Uracil (U)
Thymine (T)
Adenine (A)
Question 19:
Unlike DNA, RNA _____ (can or cannot) move through the nuclear pores and _____ (is or is not) confined to the nucleus.
→ Can
Is not
Question 20:
3 important types of RNA are _____ (which stands for _____), _____ (which stands for _____), and _____ (which stands for _____).
→ mRNA
Messenger RNA
rRNA
Ribosomal RNA
tRNA
Transfer RNA
Question 21:
A common cause of mutations in DNA is the similarity in structure between uracil and thymine. Draw their structures and circle what differentiates one from the other.
→

(Source: https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEj6od2aRe-5rGq2YDGsfjX642n1x_hdfABgk2nDtl07__kVwKU-TM5wkRiBUCAV2lUEqEKWrmte38eeKQYo3Woley4fFeqtkddfd4BR-Fr7bAchEmbtgVImVZZKF9PGhouWQLueQG-4Xp8b/s1600/uracil-thymine.png)
Question 22:
Draw the structures of Adenine, Thymine, Guanine, and Cytosine. Demonstrate which ones pair together and how they pair.
→

(Source: https://biochemistry3rst.files.wordpress.com/2014/04/hydrogen-bonds.gif)
Question 23:
In addition to forming genetic material, nucleotides also serve other purposes in the cell. Name 4 other important nucleotides and briefly describe their purposes.
→ ATP (= Adenosine TriPhosphate): main source of readily available energy for the cell
cAMP (= cyclic AMP): important component in many second messenger systems
NADH and FADH2: co-enzymes involved in Krebs cycle
PRACTICE
Question 1:
Nucleotides involve in the cell's use of _____ and comprising the building blocks of every organism's _____. They're composed of 3 components: _____, _____, and _____.
Question 2:
In DNA, the highly stable _____, along with the _____, are able to link together to form a stable and organized backbone.
Question 3:
Both sugars and phosphate groups are _____ (polar or non-polar) and, thus, can face _____ (outward or inward) into the watery solvent of the cell.
Question 4:
Nitrogenous bases can form _____ (weak, moderate, or strong) _____ bonds with each other that stabilize the _____-stranded (single or double) structure of DNA but can also be separated to allow the replication of genetic material.
Question 5:
Nucleoside = _____ sugar + _____ base + _____ (number) phosphate groups
Nucleotide = _____ sugar + _____ base + _____ (number) phosphate groups
Question 6:
Nucleotides are _____ (monomers or polymers), whereas nucleic acids are _____ (monomers or polymers). _____ (nucleotides or nucleic acids) form polymers to create _____ (nucleotides or nucleic acids).
Question 7:
Nucleotides form polymers to create nucleic acids, _____ and _____, which allow for expression of genetic traits by specifying the production of _____ (carbohydrates, proteins, or lipids).
Question 8:
In nucleic acids, nucleotides are joined together into long strands by _____ bonds between phosphate group of 1 nucleotide and 3rd carbon of pentose sugar of the other nucleotide, forming a _____ backbone.
Question 9:
Nucleotides are written in the _____ (5' to 3' or 3' to 5') direction, so that the top strand runs _____ (5' to 3' or 3' to 5') and the bottom strand runs _____ (5' to 3' or 3' to 5').
Question 10:
Name 4 nitrogenous bases and their abbreviation in DNA. Identify which 2 are purines and which 2 are pyrimidines. Identify complementary strands and how many H-bonds are formed between them, with " = " means 2 H-bonds and " ≡ " means 3 H-bonds.
Question 11:
Purine is a _____-ringed (number) structure, whereas pyrimidine is a _____-ringed (number) structure.
Question 12:
DNA usually exists in double-stranded structure described by the _____ model, named after 2 scientists who are credited with 1st theorizing DNA structure.
In double-stranded structure, AKA the B form, the 2 strands run side-by-side in opposite _____ (5' to 3' or 3' to 5') direction (thus, anti-parallel) bound together by _____ bonds between nitrogenous bases. This bonding is often referred to as _____. The length of DNA strands is measured in _____.
Question 14:
2 strands that match up in the correct order with each other are called _____ strands. When they bind together, they curl into a _____ which contains 2 distinct grooves called the _____ and the _____. Each groove spirals _____ (once or twice) around the structure for every _____ (number) base-pairs. This DNA structure is _____ (stable or unstable) in the cellular environment and allows for replication of genetic material.
Question 15:
DNA (which stands for _____) is a polymer of _____, each of which is composed of 3 parts: _____, _____, and _____. Purines are _____ and _____, whereas pyrimidines are _____ and _____.
Question 16:
_____, a nitrogenous base in RNA that is complementary to Adenine, is a _____ (purine or pyrimidine).
Question 17:
2 _____ (phosphodiester bonds or H-bonds) hold Adenine and Thymine together, whereas 3 _____ (phosphodiester bonds or H-bonds) hold Guanine and Cytosine together. This means that more energy is required to separate _____ (Adenine = Thymine bond or Guanine ≡ Cytosine bond).
Question 18:
RNA (which stands for _____) is identical to DNA (which stands for _____) in structure except that:
1. Carbon number _____ on pentose sugar is not _____, meaning that it has a _____ group attached;
2. RNA is almost always _____-stranded (single or double); and
3. RNA contains the pyrimidine _____ instead of _____, both of which are complementary to _____.
Question 19:
Unlike DNA, RNA _____ (can or cannot) move through the nuclear pores and _____ (is or is not) confined to the nucleus.
Question 20:
3 important types of RNA are _____ (which stands for _____), _____ (which stands for _____), and _____ (which stands for _____).
Question 21:
A common cause of mutations in DNA is the similarity in structure between uracil and thymine. Draw their structures and circle what differentiates one from the other.
Question 22:
Draw the structures of Adenine, Thymine, Guanine, and Cytosine. Demonstrate which ones pair together and how they pair.
Question 23:
In addition to forming genetic material, nucleotides also serve other purposes in the cell. Name 4 other important nucleotides and briefly describe their purposes.
ANSWER KEY
Question 1:
→ Energy
Genetic material
A 5-carbon (pentose) sugar
A nitrogenous base
A phosphate group
**Review**
(Source: http://image.slidesharecdn.com/organicchemistry-131105135539-phpapp02/95/organic-chemistry-7-638.jpg?cb=1383659789)
Question 2:
→ Sugars
Phosphate groups
Question 3:
→ Polar
Outward
Question 4:
→ Weak
Hydrogen
Double
Question 5:
→ Pentose
Nitrogenous
0
Pentose
Nitrogenous
1 or more
Question 6:
→ Monomers
Polymers
Nucleotides
Nucleic acids
Question 7:
→ DNA (DeoxyriboNucleic Acid)
RNA (RiboNucleic Acid)
Proteins
Question 8:
→ Phosphodiester
Sugar-phosphate
Question 9:
→ 5' to 3'
5' to 3'
3' to 5'
Question 10:
→ Adenine (A) = Thymine (T)
Guanine (G) ≡ Cytosine (C)
**Mnemonic**
Pyrimidine: cytosine and thymine
Question 11:
→ 2
1
Question 12:
→ Watson-Crick
→ 3' to 5'
Hydrogen
Base-pairing
Base-pair (bp)
Question 14:
→ Complementary
Double helix
Major groove
Minor groove
Once
10
Stable
(Source: http://www.biologynoteshelp.com/wp-content/uploads/2016/06/MAJOR-AND-MINOR-GROOVES-210x300.jpg)
(Source: https://classconnection.s3.amazonaws.com/251/flashcards/704251/png/grooves1316409303998.png)
Question 15:
→ DeoxyriboNucleic Acid
Nucleotides
Phosphate group
5-carbon (pentose) sugar
Question 16:
→ Uracil
Pyrimidine
Question 17:
→ H-bonds
H-bonds
Guanine ≡ Cytosine bond
Question 18:
→ RiboNucleic Acid
DeoxyriboNucleic Acid
2
Deoxygenated
Hydroxyl
Single
Uracil (U)
Thymine (T)
Adenine (A)
Question 19:
→ Can
Is not
Question 20:
→ mRNA
Messenger RNA
rRNA
Ribosomal RNA
tRNA
Transfer RNA
Question 21:
→

(Source: https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEj6od2aRe-5rGq2YDGsfjX642n1x_hdfABgk2nDtl07__kVwKU-TM5wkRiBUCAV2lUEqEKWrmte38eeKQYo3Woley4fFeqtkddfd4BR-Fr7bAchEmbtgVImVZZKF9PGhouWQLueQG-4Xp8b/s1600/uracil-thymine.png)
Question 22:
→

(Source: https://biochemistry3rst.files.wordpress.com/2014/04/hydrogen-bonds.gif)
Question 23:
→ ATP (= Adenosine TriPhosphate): main source of readily available energy for the cell
cAMP (= cyclic AMP): important component in many second messenger systems
NADH and FADH2: co-enzymes involved in Krebs cycle
Subscribe to:
Posts (Atom)











